Make yourself familiar with the instruments & glassware (hover for more info)
 Measuring cylinder
Measuring cylinder
 Conical Flask
Conical Flask
 Weighing Scale
Weighing Scale
 Hot plate
Hot plate
 Burette
Burette
Step 1: Select the sample




Which of the sample has darkest color?
Step 2: Weigh 2gms of oil sample in conical flask
 click to place flask
click to place flask

What is the required quantity of oil to be taken for % FFA content?
Step 3: Add 50 ml ethyl alcohol in conical flask
 click to pour
alcohol
click to pour
alcohol


What is the required quantity of oil to be taken for % FFA content?
Step 4: Heat the mixture upto boiling condition
 click to turn on hot
plate
click to turn on hot
plate
Step 5: Add 2-3 drops of phenolpthalein indicator in hot condition

Step 6: Titrate the mixture with 0.1 N NaOH solution

0 ml
 click to pour
0.1N NaOH
click to pour
0.1N NaOH
Which type of titration has been used to determine % FFA
Observation Table
| Selected sample | Weight of sample (g) (W) | Normality of NaOH (N) | Burette reading (ml) | Titrant used (ml) | |
|---|---|---|---|---|---|
| Initial value | Final value | ||||
| 2.0 | 0.1 | 0.0 | |||
FFA(%) =
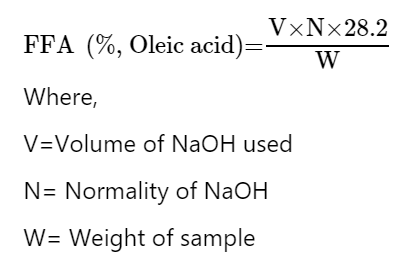
| FFA (%, Oleic acid) = | V x N x 28.2 |
|---|---|
| W | |
| FFA (%, Oleic acid) = | x 0.1 x 28.2 |
| 2 | |
| FFA (%, Oleic acid) = |
Inference
The maximum permissible limit of FFA as per FSSAI in the edible oil is 0.5%
The limit of FFA as per FSSAI standard is 0.05%. The FFA content of the given sample is 0.28%.
So, this sample is in the FSSAI range and safe for edible purposes.