Objective:
To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as acatalyst.
To determine the rate constant of the hydrolysis of Ethyl acetate using an acid as acatalyst.
1.Add Appratus by clicking button named ADD Appratus
2.Select a Reagent Bottle & HCL.
3.Select Ethyl Acetate and add into Reagent Bottle
4.Stirr the mixture
5.Add 10(ml) of mixture in 100(ml) conical flask containing ice.
6.In Solution add some drops of phenolphthalein Indicator.
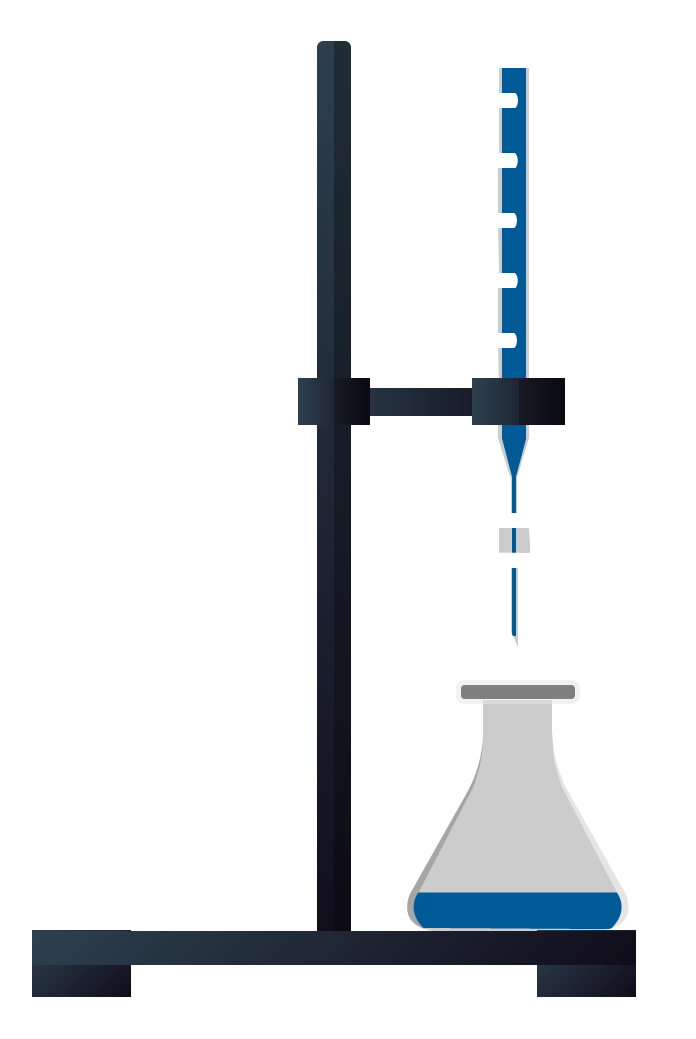
7.Bring Conical Flask under the burette.
8.Fill the burete with NAOH
9.Start adding NaOH drop until color of the solution become pink.
10.After getting pink color repeat steps 4 to 9 five times after 10 minutes
11.Remaining solution to be kept on water bath at 60.c for 10 min and cool down then repeat steps for calculating v∞
12.Repeat steps again and find the value


| Time (min) | Volume of solution | Titre value(ml) | (V∞-Vt) | Log((V∞ - Vt) | Rate of Constant(k) |
|---|---|---|---|---|---|
| 0 | 10 | 0.2(V0) | 19.1 | 1.28 | 19.1 (ingnored) |
| 10 | 10 | 5.0 | 14.3 | 1.155 | 0.288 |
| 20 | 10 | 7.2 | 12.1 | 1.083 | 0.281 |
| 30 | 10 | 11.1 | 8.2 | 0.914 | 0.25 |
| 40 | 10 | 13.2 | 6.1 | 0.785 | 0.28 |
| 60 | 10 | 19.3(V∞) | -- | -- | -- |
V0:Initial Volume
Vt:Volume at time "t"
V∞:Volume at time 60 min
Calculate Rate Contant(k) using this formula :(2.303/t) * ( log((V∞-V0)/(V∞-Vt)))
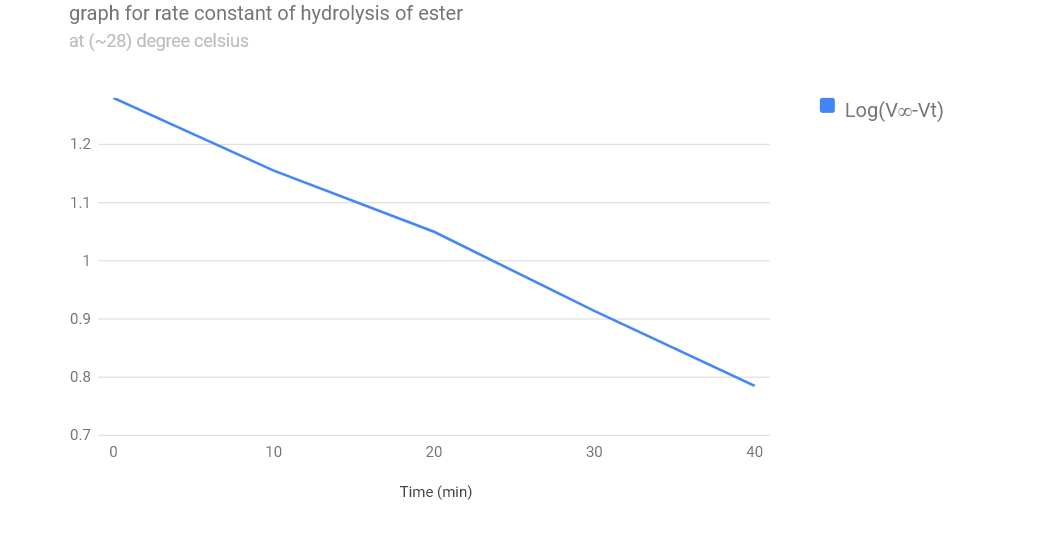
In conclusion ,it can be seen from this experiment that rate of reaction is concentation dependent while rate constant is not.Also ,that the rate constant for the hydrolysis of ethyl acetate with sodium hydroxide using HCl as a catalyst at 28 C is approximately 0.274min-1
The rate constant for the hydrolysis of an easter from- 1.Calculated value= 0.274
